 Mini Review
Mini Review
Use of Sea Water as Fertilizer
Dr Fakhraddin Abasov* and Guliyev AG
Department of Soil Science and Agrochemistry, Azerbaijan National Academy of Sciences, Azerbaijan.
Fakhraddin Abasov, Dr of Philosophy of Chemical Sciences, Department of Soil Science and Agrochemistry, National Academy of Sciences , Azerbaijan, AZ1073, M. RAHİM Street 5, Baku, Azerbaijan;
Received Date: January 26, 2022; Published Date: February 17, 2022
Abstract
The use of sea water as fertilizer as well as irrigation water opens up new opportunities in not only the world economy but also agricultural areas. From this point of view, the use of sea water for this purpose remains an actual problem. As you know, nowadays the majority of world countries suffer from thirst.
Keywords: Osmos; Sea water; Water purification; Agricultural; Irrigation; Fertilization; Semiconducting membranes
Introduction
Therefore, in case the sea water is used as useful water source, it will contribute to the solution of some problems. Some countries in the world already use the sea water as a useful source of water. With this osmosis method, it is carried out under high pressure by means of semiconducting membranes. The disadvantage of this method is high energy consumption and the very large cost. Also, in some countries this method is based on the evaporation of sea water from salts and this causes high energy consumption. For this purpose, we can use solar energy in order to decrease energy consumption [1]. As you know, as a result of sea water analysis, the most harmful ions have been determined inside it. Meanwhile, sea water consists of large amount of harmful microorganisms for plants. That is why, it is the purpose to neutralize sea water both chemically and biologically.
For this purpose, in order to solve the problem, physical and chemical methods are utilized to make sea water useful.
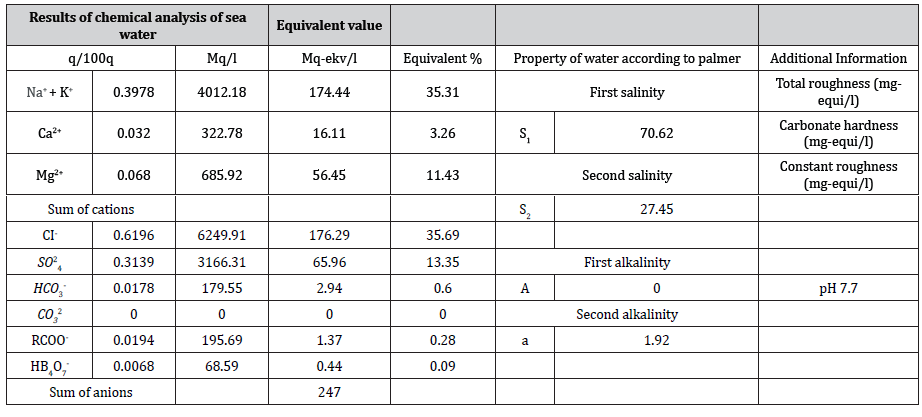
First of all, lets get acquainted with ions that have been determined inside sea water as a result of analysis. This analysis was conducted upon MS-1669347-05-04 standard method (Table 1).
Table 1:
Heavy Metals ICP-OES (inductively Coupled plasma-optical emission spectrometer)
Table 2:
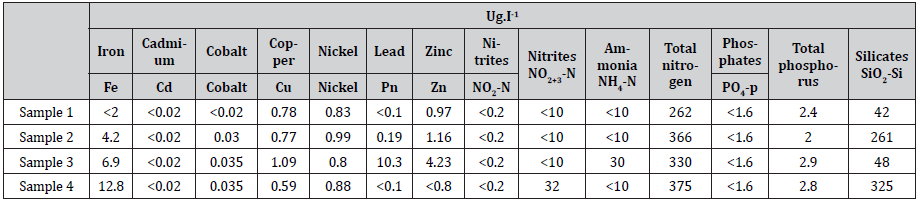
(Table 2) As shown in the table above, as sea water consists of large amount of CI- and 2 4 SO− ions, this prevents the use of water in plants [2]. Therefore, it is firstly required to neutralize or disable CI- and so-24ions in the sea water.
It is conducted by doing the below mentioned conversions (equations).
KCL+Pb(NO3)2 == KNO3+PbCL2↓
NaCL+Pb(NO3)2 === NaNO3+PbCL2↓
Na2SO4+Pb(NO3)2 == NaNO3+PbSO4↓
K2SO4+Pb(NO3)2 ==== KNO3+PbSO4↓
As you see from reaction equation, Cl- and 2 so-24ions are released in the form of sediments, KNO3 and NaNO3 remains in water and by this way, we get nitrogen, phosphorus and potassium from sea water, which are the main nutrients for plants.
The rest of ions are both few and it is meaningless to neutralize them because this small number of ions are beneficial for plants [3].
The microorganisms in sea water are neutralized with special physical methods. We have already used the purified water we got and obtained really good results. As the rest of ions and the ions that entered into the environment as a result of reaction ( 3 NO − ) are used as fertilizer, we can use sea water as fertilizer and from this point of view, we can utilize sea water both for irrigation and fertilizer.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Sekino Massaki, Gotoh Katsuji, Janaga Joichiro, Nikko Hiroshi Matsunaga Kazuhiko (1985) Reverse osmosis modules for water desalination. Chem Eng Progr 81(12): 52-56.
- Aly G All-Haddad, Abdel-Jawad (1987) M Parametric study on falling-film sea water desalination. Desalinayion 65: 43-55.
- Vinakov NA, Trofimov VN, Smirnov EM (1988) Chelyabinsk branch of the All-Russian Research Institute of Water Supply Sewerage. Hydraulic structures and engineering geodesy.
-
Dr Fakhraddin Abasov, Guliyev AG. Use of Sea Water as Fertilizer. Ad Oceanogr & Marine Biol. 3(2): 2022. AOMB. MS.ID.000556.
-
Osmos, Sea water, Water purification, Agricultural, Irrigation, Fertilization, Semiconducting membranes
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






